Last updated: 26th April 2023
This page will only be updated if Viatris anticipates stock constraint at patient level.
Therefore, this page will not be updated if there is no change in the supply of stock. However, stock levels are monitored weekly.
The date updated reflects the last time the page was changed
| MONO FORMULATION OPTIONS (17ß ESTRADIOL) | |
|---|---|

|
Elleste™ Solo
Doses:

|

|
Zumenon®
Doses:

|

Dear Healthcare Professional,
Earlier this year, Viatris informed you that our third-party manufacturing partner was experiencing an interruption in the production of Elleste™, which included; Elleste Solo™ (1mg & 2mg), Elleste Duet™ (1mg & 2mg) and Elleste Duet™ Conti (2mg).
At that time, we anticipated a temporary interruption and for stock levels to resume during the middle of 2019. To minimise disruption to patients and to support pharmacies, societies and healthcare professionals (HCPs) across the country we took several proactive steps, including:
- • sending two Elleste™ stock constraint mailers to 50,000+ HCPs;
- • informing the British Menopause Society;
- • continuously communicating with the National Pharmacy Association; and
- • creating a MyWay Pharmacist leaflet for our salesforce to use when visiting pharmacists across the country.
In addition, we created a new promotional page on our dedicated HCP website, called MyWay HRT Overview and Stock Availability. This page provides details of our range of HRT products, stock availability and options to switch patients to alternative products where appropriate.
To ensure the stable supply of Elleste™ Oral Tablets in the future, Viatris will be transferring the production of Elleste™ to a Viatris owned and operated facility.
The site transfer is in progress and we anticipate production to resume by second half of 2020. In the meantime, any stock of Elleste™ Oral Tablets in the market, is available to order and prescribe. Once that supply has been depleted, the replenishment of Elleste™ Oral Tablets will not be available until the site transfer process has been completed.
We will continue to support pharmacies, societies and healthcare professionals by providing updates to our materials and communications including updates to stock availability, timing for resolution and information about Viatris other HRT products.
Please note that this does not affect the transdermal patches; Elleste Solo™ MX (40 and 80mcg).
Information for Prescribers
Viatris is unable to make any specific treatment recommendations to healthcare professionals or individual patients. We would like to highlight that there are other Hormone Replacement Therapy products in our portfolio which are available, please visit mywayhub.co.uk/en-gb/range or www.mylanconnects.co.uk for more information. Please note these are promotional websites and contain information on Viatris products.
For enquiries in relation to availability of a product, please contact our Customer Service team on, +44 (0)1707 853 100 or mguk_customer.service@mylan.co.uk. For medical information enquiries, please contact Viatris medical information team on +44 (0)1707 853000 (option 1) or info.uk@mylan.co.uk.
Yours Sincerely,
Dr Ken Tam
Senior Medical Advisor
About Elleste™
Elleste is a hormone replacement therapy (HRT) for oestrogen deficiency symptoms in post and peri-menopausal women.
Resources
Elleste Solo™ 1mg – Patient Information Leaflet and SmPC
Elleste Solo™ 2mg – Patient Information Leaflet and SmPC
Elleste Duet™ 1mg – Patient Information Leaflet and SmPC
Elleste Duet™ 2mg – Patient Information Leaflet and SmPC
Elleste Duet™ Conti 2mg – Patient Information Leaflet and SmPC
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Viatris by phone: 0800 121 8267 or email: ukpharmacovigilance@mylan.com
ELL-2019-0050
Date of preparation: August 2019
For Medical Information please e-mail info.uk@viatris.com
For Customer Service please e-mail productenquiries@viatris.com

-
The Femoston® range of products contain both 17ß estradiol and
dydrogesterone - The Femoston® range offers both sequential and continuous combined options
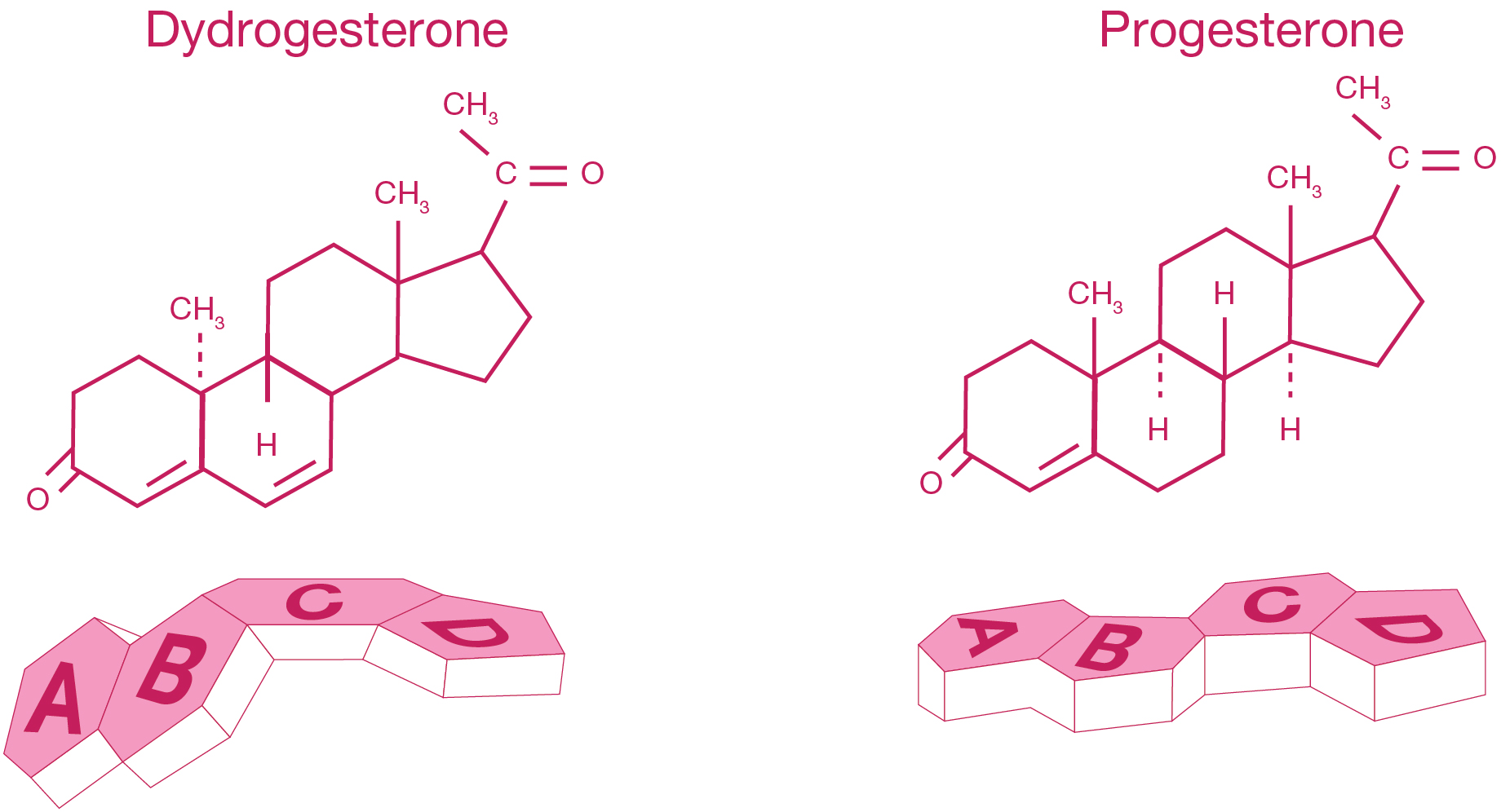
- Dydrogesterone is a stereoisomer of progesterone with an additional double bond between carbons 6 and 71
- Dydrogesterone’s molecular structure and shape makes it a highly selective progestogen, binding almost exclusively to the progestogen receptors1
|
Progestogen
|
Progestogenic
|
Estrogenic
|
Androgenic
|
Anti-androgenic
|
Glucocorticoid
|
Anti-
mineralo-corticoid |
|---|---|---|---|---|---|---|
| Progesterone | + | – | – | ± | + | + |
| Dydrogesterone | + | – | – | ± | – | ± |
| Drospirenone | + | – | – | + | – | + |
| MPA* | + | – | ± | – | + | – |
| Norethisterone | + | + | + | – | – | – |
| Levonorgestrel | + | – | + | – | – | – |
+ Effective; ± Weakly effective; – Not effective
*MPA: medroxyprogesterone acetate
| Receptors | Common side effects by stimulation of receptors |
|---|---|
| Estrogenic | Breast tenderness, enlargement, leg cramps, bloating, nausea, headache |
| Progestogenic | PMS type symptoms, mood changes |
| Androgenic | Oily skin, acne, hirsutism |
| Glucocorticoid | Dosage and duration dependent: oedema, fluid retention, weight gain |
| Mineralcorticoid3 | Oedema, weight gain, bloating and migraine |
Why go low?
These estimated dose equivalents are subject to significant individual variations in absorption and metabolism. Doses refer to oral therapy unless otherwise specified.
| Estrogen | Ultra Low | Low | Standard | High |
|---|---|---|---|---|
| Conjugated equine estrogens (mg) |
0.3 | 0.625 | 1.25 | |
| Micronized 17ß-estradiol (mg) |
0.5 | 1 | 2 | 4 |
| Estradiol valerate (mg) |
1 | 2 | ||
| Transdermal 17ß-estradiol (μg) |
25 | 50 | 100 |
Table adapted from Maturitas, 40, Gambacciani M. Hormone replacement therapy: the benefits
in tailoring the regimen and dose. 195-201, Copyright (2001), with permission from Elsevier.
- Both the International Menopause Society (IMS) and the British Menopause Society (BMS) recommended that women should be placed on the lowest effective doses5,7,8
-
Some benefits of going low.6-8
- Provides effective symptom relief
- High rate of amenorrhoea (91% of women bleed free after 10-12 months of use) and good tolerability
- Ultra-low dose continuous combined estradiol and progestogen regimens (e.g. 0.5mg estradiol in combination with dydrogesterone 2.5 mg) appear to maintain the benefits of higher dose regimens whilst allowing minimal use of progestogen to reduce side-effects
-
References
- 1) Schindler AE, Campagnoli C, Druckmann R, et al. Classification and pharmacology of progestins. Maturitas. 2008;61(1–2):171–80.
- 2) Menopause matters, URL: https://www.menopausematters.co.uk/sideeffects.php.
- 3) Jones EE. Androgenic effects of oral contraceptives: implications for patient compliance. Am J Med.1995;16;98(1A):116S-119S. doi: 10.1016/s0002-9343(99)80069-2.
- 4) Panay N, et al. Progestogen intolerance and compliance withhormone replacement therapy in menopausal women. Human Reproduction Update. 1997;3(2):159-171.
- 5) Baber RJ, Panay N, Fenton AT. 2016 IMS Recommendations on women’s midlife health and menopause hormone therapy. Climacteric.16 Mar;19(2):109-50.
- 6) Stevenson J, et al.Oral ultra-low dose continuous combined hormone replacement therapy with 0.5 mg 17β-oestradiol and 2.5 mg dydrogesterone for the treatment of vasomotor symptoms: results from a double-blind, controlled study. Maturitas. 2010 Nov;67(3):227-32.
- 7) Panay N, et al. on behalf of the British Menopause Society (BMS) and Women’s Health Concern (WHC). Menopause International. 2013; 19(2): 59-68.
- 8) Hamoda H, et al. The British Menopause Society & Women's Health Concern 2020 recommendations on hormone replacement therapy in menopausal women. Post Reprod Health. 2020;26(4):181-209.
- 9) Viatris data on file 2021.
HCP Disclaimer
This website is intended for UK healthcare professionals only.
Viatris Connect is an online platform for UK healthcare professionals.
Across the website you will find news, blogs and product information.
FREE Menopause and HRT webinars available to watch by registering to Viatris Connect today
Please note that the website contains promotional and non-promotional material including educational content and resources to help you and your patients.
REGISTER NOW








