
Femoston®
(estradiol + dydrogesterone)
*IQVIA Midas via SMART worldwide 2019, Femoston® launch date.
What is Femoston®?
Femoston® contains estrogen and progestogen. Estrogen relieves estrogen deficiency symptoms such as hot flushes. Progestogen provides endometrial protection for non-hysterectomised women.
Menopausal women experience a drop in their estrogen levels, hormone replacement therapy (HRT) can be advised to alleviate the symptoms associated with estrogen deficiency and improve their overall quality of life.1

Femoston® is a hormone replacement therapy (HRT), which is suitable for postmenopausal women who require estrogen replacement therapy and have not had their uterus removed (hysterectomy). During the menopausal transition levels of estrogen in a woman’s body start to decline, causing vasomotor symptoms like hot flushes.2
The Femoston® range - the only HRT to contain Dydrogesterone
- The Femoston® range of products contain both 17β-estradiol and dydrogesterone
- The Femoston® range offers both sequential and continuous combined options
- Dydrogesterone is a stereoisomer of progesterone with an additional double bond between carbons 6 and 73
- Dydrogesterone’s molecular structure and shape makes it a highly selective progestogen, binding almost exclusively to the progestogen receptors3
Composition of Femoston®
Femoston® is a combination of 17β-estradiol and dydrogesterone. Femoston® is available in 4 preparations with estradiol / dydrogesterone in doses of 0.5 mg/2.5 mg and 1 mg/5 mg as continuous combined preparations and 1 mg/10 mg and 2 mg/10 mg as sequential combined preparations.4-7
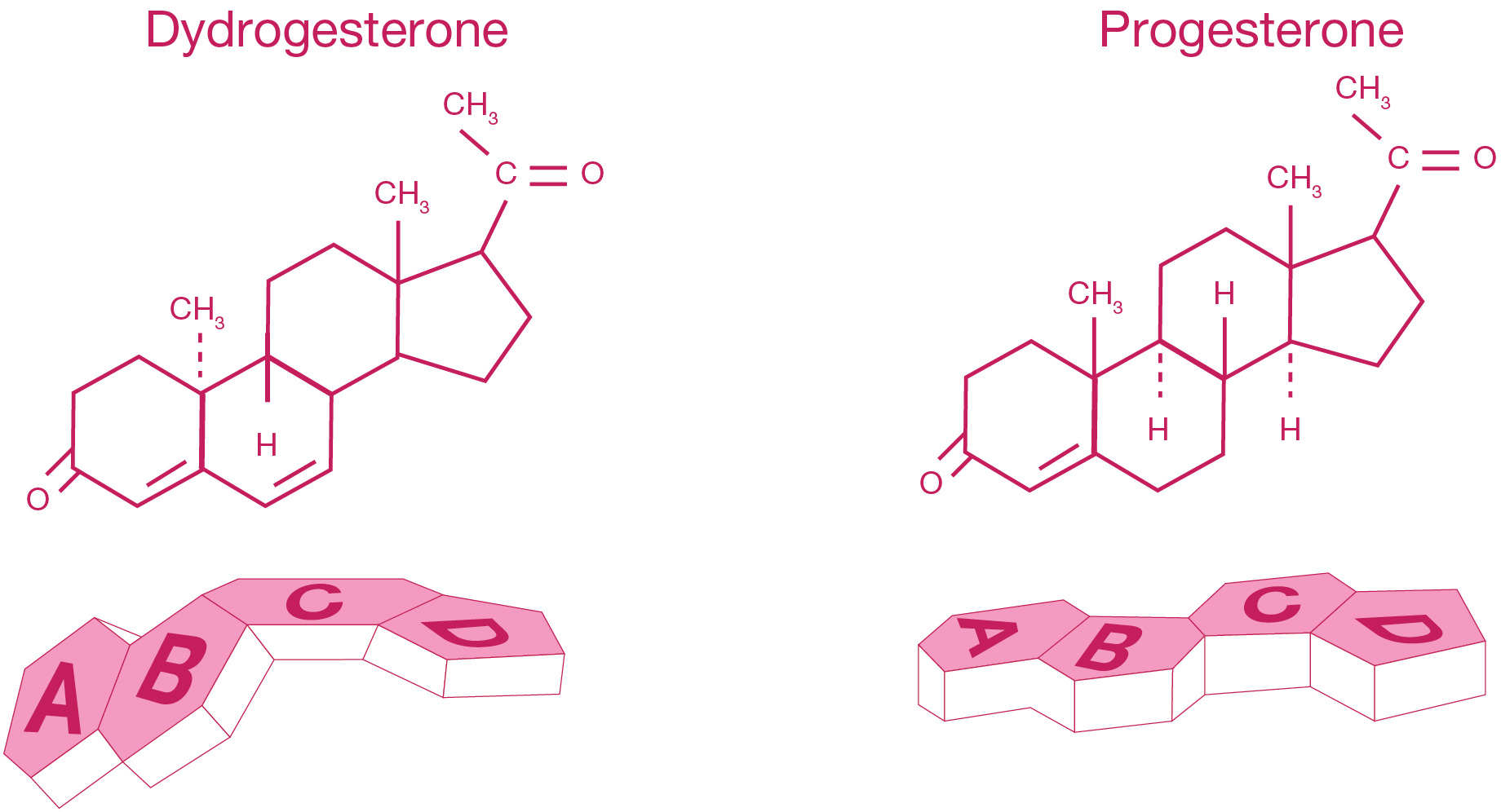
Adapted from Fig 1 Schindler AE. Progestational effects of dydrogesterone in vitro, in vivo and on the human endometrium. Maturitas 2009;65(Suppl 1):S3-S11.
Benefits of combination therapy of estradiol and dydrogesterone
The addition of a progestogen cyclically for at least 12 days per month/28 day cycle or continuous combined estrogen-progestogen therapy in non-hysterectomised women prevents the excess risk of endometrial hyperplasia and carcinoma associated with estrogen-only HRT.4 According to a systematic review of literature, 17β-estradiol/dydrogesterone combinations in doses of 0.5 mg/2.5 mg, 1 mg/5 mg, 1 mg/10 mg and 2 mg/10 mg were associated with a statistically significant improvement in the postmenopausal symptoms.10 Furthermore, studies have demonstrated that combination of 17β-estradiol/dydrogesterone in doses of 1 mg/10 mg, 2 mg/10 mg and 1 mg/5 mg can help as a second line agent in the prevention of osteoporosis as evaluated by the assessment of bone mineral density (BMD).11,12
Read more about the indications of Femoston® & Femoston®-conti below.
Benefits of the ultra-low dose
Lower doses of combined HRT may be associated with reduced incidences of breast tenderness and higher rates of amenorrhoea compared to combined HRT regimens with higher doses estrogen.8,9
A randomised controlled trial with 0.5 mg/2.5 mg and 1 mg/5 mg estradiol/dydrogesterone for a period of 13 weeks reported a significant mean reduction in the number of hot flushes from baseline as compared with placebo.8
Treatment with Femoston® conti (0.5 mg/2.5 mg) ultra low dose continuous combined therapy demonstrated a high rate of amenorrhoea (91% of women bleed free by months 10-12) and a good tolerability profile.8
Additionally, ultra-low dose continuous combined estradiol and progestogen regimens (e.g. 0.5 mg estradiol in combination with dydrogesterone 2.5 mg) appear to maintain the benefits of higher dose regimens whilst allowing minimal use of progestogen to reduce side-effects.13
The table below provides a rough estimate of equivalent doses. These dose equivalents are subject to significant individual variations in absorption and metabolism. Preparations are oral, unless otherwise specified.
| Estrogen | Ultra Low | Low | Standard | High |
|---|---|---|---|---|
| Conjugated equine estrogens | 0.3 | 0.625 | 1.2 | |
| Micronized 17β-estradiol (mg) | 0.5 | 1 | 2 | 4 |
| Estradiol valerate (mg) | 1 | 2 | ||
| Transdermal 17β-estradiol (mcg/24hr) | 25 | 50 | 100 |
195-201, Copyright (2001), with permission from Elsevier.14
Indications of Femoston®
Femoston® is indicated for estrogen deficiency symptoms in postmenopausal women at least 6 months since last menses and Femoston®-conti is indicated for estrogen deficiency symptoms in postmenopausal women at least 12 months since last menses.4-7 Femoston® 1/10, 2/10 and Femoston®-conti 1/5 are indicated for the prevention of osteoporosis in postmenopausal women at high risk of future fractures who are intolerant of, or contraindicated for, other medicinal products approved for the prevention of osteoporosis.4,5,7
Side effects of Femoston®
The frequency of side effects of Femoston® as observed from clinical trials may range from very common to rare. The most common side effects include headache, abdominal pain, back pain and breast tenderness. The common side effects can include vaginal candidiasis, depression, nervousness, migraine, dizziness, nausea, vomiting, flatulence; allergic skin reactions, menstrual disorders, weight gain, cervical discharge and pelvic pain. Please refer to the Summary of Product Characteristics for full information.4-7
Progestogenic Side Effect Profiles
Femoston® and Femoston®-conti contains 17β-estradiol and dydrogesterone.4-7 Dydrogesterone does not stimulate the following receptors: estrogenic, androgenic or glucocorticoid.3,15,16
|
Progestogen
|
Progestogenic
|
Estrogenic
|
Androgenic
|
Anti-
androgenic |
Glucocorti
coid |
Anti-
mineralo-corticoid |
|---|---|---|---|---|---|---|
| Progesterone | + | - | - | ± | + | + |
| Dydrogesterone | + | - | - | ± | - | ± |
| Drospirenone | + | - | - | + | - | + |
| MPA* | + | - | ± | - | + | - |
| Norethisterone | + | + | + | - | - | - |
| Levonorgestrel | + | - | + | - | - | - |
+ Effective; ± Weakly effective; – Not effective
*MPA: medroxyprogesterone acetate
-
References
- 1) Baber RJ, Panay N, Fenton AT. 2016 IMS Recommendations on women’s midlife health and menopause hormone therapy. Climacteric.16 Mar;19(2):109-50.
- 2) Rossmanith WG, Ruebberdt W. What causes hot Flushes? The neuroendocrine origin of vasomotor symptoms in the menopause. Gynecol Endocrinol. 2009 May;25(5):303-14.
- 3) Schindler AE, Campagnoli C, Druckmann R, et al. Classification and pharmacology of progestins. Maturitas. 2008;61(1–2):171–80.
- 4) Femoston® 1mg/10mg Summary of Product Characteristics.
- 5) Femoston® 2/10mg. Summary of Product Characteristics.
- 6) Femoston® Conti 0.5mg/2.5mg Summary of Product Characteristics.
- 7) Femoston® Conti 1mg/5mg Summary of Product Characteristics.
- 8) Stevenson JC, Durand G, Kahler E, Pertyński T. Oral ultra-low dose continuous combined hormone replacement therapy with 0.5 mg 17β-oestradiol and 2.5 mg dydrogesterone for the treatment of vasomotor symptoms: results from a double-blind, controlled study. Maturitas. 2010 Nov;67(3):227-32.
- 9) Bergeron C et al. Ultra low dose continuous combined hormone replacement therapy with 0.5mg 17beta-oestradiol and 2.5mg dydrogesterone: protection of the endometrium and amenorrhoea rate. Maturitas. 2010 Jun;66(2):201-5.
- 10) Stevenson JC, Panay N, Pexman-Fieth C. Oral estradiol and dydrogesterone combination therapy in postmenopausal women: review of efficacy and safety. Maturitas. 2013 Sep;76(1):10-21.
- 11) Stevenson JC, Teter P, Lees B. 17beta-estradiol (1 mg/day) continuously combined with dydrogesterone (5, 10 or 20 mg/day) increases bone mineral density in postmenopausal women. Maturitas. 2001;38:197-203.
- 12) Lees BA, Stevenson JC. The prevention of osteoporosis using sequential low-dose hormone replacement therapy with estradiol-17β and dydrogesterone. Osteoporos Int. 2001 May;12(4):251-8.
- 13) Hamoda H et al. The British Menopause Society & Women’s Health Concern 2020 recommendations on hormone replacement therapy in menopausal women. Post Reproductive Health. October 2020.
- 14) Gambacciani M, Genazzani AR. Hormone replacement therapy: the benefits in tailoring the regimen and dose. Maturitas. 2001;40(3):195-201.
- 15) Menopause Matters. HRT side effects. Available at: www.menopausematters.co.uk/sideeffects.php (accessed September 2021).
- 16) Panay N, et al. Progestogen intolerance and compliance with HRT in menopausal women. Human Reproduction Update 1997; 3(2): 159-171.

Zumenon®
Zumenon® is indicated for estrogen deficiency symptoms in postmenopausal women at least 6 months since last menses.

MyWebinars
Click here to listen and watch webinars discussing the latest information on menopause and HRT, presented by national and international experts.
HCP Disclaimer
This website is intended for UK healthcare professionals only.
Viatris Connect is an online platform for UK healthcare professionals.
Across the website you will find news, blogs and product information.
FREE Menopause and HRT webinars available to watch by registering to Viatris Connect today
Please note that the website contains promotional and non-promotional material including educational content and resources to help you and your patients.
REGISTER NOW


